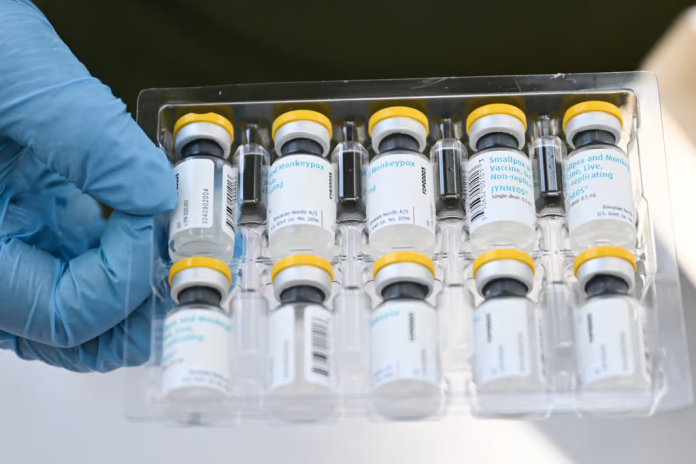
The World Health Organisation (WHO) stated that it had granted the first authorisation for the use of mpox vaccine for adults, describing it as an important step towards combating the disease in Africa and beyond.
WHO Director-General Tedros Adhanom Ghebreyesus declared:
This first pre-qualification of a vaccine against mpox is an important step in our fight against the disease, both in the context of the current outbreaks in Africa, and in future.
Pre-qualification of the Bavarian Nordic A/S vaccine means that donors, such as GAVI, Vaccine Alliance, and UNICEF, can buy it. However, supplies are limited as there is only one manufacturer.
Tedros called for an “urgent” increase in procurement, donations, and deployment of the vaccine where it is most needed.
The WHO approval allows the vaccine to be administered to people 18 years of age and older in a two-dose regimen. The approval states that while the vaccine is not currently licensed for people under 18, it can be used for infants, children, and adolescents “in outbreak settings where the benefits of vaccination outweigh the potential risks.”
Last month, the African Centre for Disease Control and Prevention said nearly 70% of cases in Congo were in children under 15, who also accounted for 85% of deaths. The centre also said on Thursday that 107 new deaths and 3,160 new cases had been reported in the past week.
Mpox belongs to the same virus family as smallpox but causes milder symptoms such as fever, chills, and body aches.